Among US patients 6+ months of age:
- ≈2.6 million remain uncontrolled despite treatment2-4,b
- ≈1 million uncontrolled patients have not seen an eczema specialist3,c,d

JEN HAS LESS ITCH AND CLEARER
SKIN IN HER FAVORITE PARTY DRESS
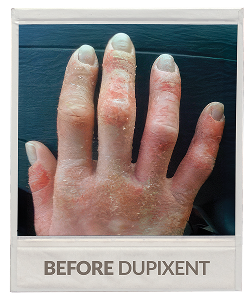

Real patient being treated with DUPIXENT.
Individual results may vary.
Real patient being treated with DUPIXENT.
Individual results may vary.



KAYDEN HAS LESS ITCH AND CLEARER
SKIN AS HE MAKES A SPLASH

explore a real adolescent’s story
Real patient being treated with DUPIXENT.
Individual results may vary.
Real patient being treated with DUPIXENT.
Individual results may vary.



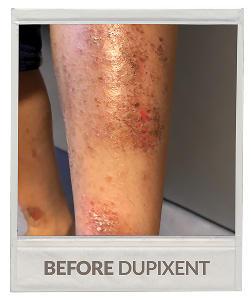
BAILEY HAS LESS ITCH AND CLEARER
SKIN DURING STORYTIME


Real patient being treated with DUPIXENT.
Individual results may vary.
Real patient being treated with DUPIXENT.
Individual results may vary.


When topical Rx therapies are not enough
DUPIXENT is the first and only biologic approved from infancy to adulthood for uncontrolled moderate-to-severe atopic dermatitis
Strong recommendation for the use of dupixent in aad ad guidelines for appropriate uncontrolled moderate-to-severe AD patients1,a
Despite treatment, many patients with moderate-to-severe atopic dermatitis may have difficulty achieving adequate control2

Among US patients 6+ months of age:

Patients can be disrupted by unpredictable flares—day
and night5,6

Atopic dermatitis places a burden on children’s family and caregivers
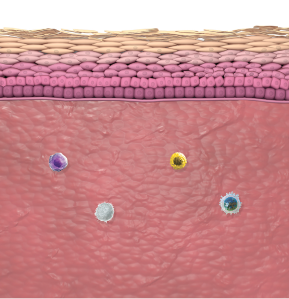
In atopic dermatitis, nonlesional skin is not normal skin8-10
Healthy skin is free from inflammatory activity
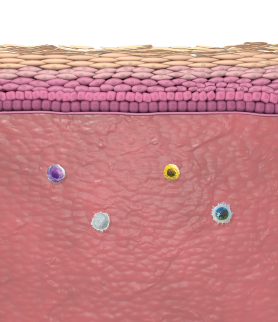
Inflammatory activity and a compromised barrier are present
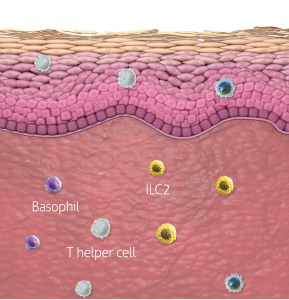
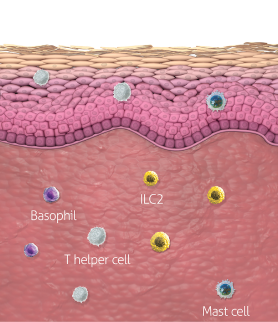

A systemic treatment like DUPIXENT that targets a cause of underlying type 2 inflammation may be helpful for managing uncontrolled moderate-to-severe atopic dermatitis1,11
The mechanism of dupilumab action has not been definitively established.
See How DUPIXENTDUPIXENT MyWay® helps ensure patients have access to DUPIXENT and are provided with assistance in navigating the insurance process
Learn more about Dupixent MyWayAtopic Dermatitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 6 months and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids.
Asthma: DUPIXENT is indicated as an add-on maintenance treatment of adult and pediatric patients aged 6 years and older with moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid dependent asthma. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm or status asthmaticus.
Chronic Rhinosinusitis with Nasal Polyps: DUPIXENT is indicated as an add-on maintenance treatment in adult and pediatric patients aged 12 years and older with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).
Eosinophilic Esophagitis: Dupixent is indicated for the treatment of adult and pediatric patients aged 1 year and older, weighing at least 15 kg, with eosinophilic esophagitis (EoE).
Prurigo Nodularis: DUPIXENT is indicated for the treatment of adult patients with prurigo nodularis (PN).
Chronic Obstructive Pulmonary Disease: DUPIXENT is indicated as an add-on maintenance treatment of adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm.
Chronic Spontaneous Urticaria: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 12 years and older with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. Limitations of Use: DUPIXENT is not indicated for treatment of other forms of urticaria.
Bullous Pemphigoid: DUPIXENT is indicated for the treatment of adult patients with bullous pemphigoid (BP).
CONTRAINDICATION: DUPIXENT is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients.
WARNINGS AND PRECAUTIONSHypersensitivity: Hypersensitivity reactions, including anaphylaxis, acute generalized exanthematous pustulosis (AGEP), serum sickness or serum sickness-like reactions, angioedema, generalized urticaria, rash, erythema nodosum, and erythema multiforme have been reported. A case of AGEP was reported in an adult subject who participated in the bullous pemphigoid development program. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue DUPIXENT.
Conjunctivitis and Keratitis: Conjunctivitis and keratitis occurred more frequently in AD, COPD, and BP subjects who received DUPIXENT versus placebo, with conjunctivitis being the most frequently reported eye disorder in AD. Conjunctivitis also occurred more frequently in adult CRSwNP and PN subjects who received DUPIXENT compared to those who received placebo. Conjunctivitis and keratitis have been reported with DUPIXENT in postmarketing settings, predominantly in AD patients. Some patients reported visual disturbances (e.g., blurred vision) associated with conjunctivitis or keratitis. Advise patients or their caregivers to report new-onset or worsening eye symptoms. Consider ophthalmological examination for patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis, as appropriate.
Eosinophilic Conditions: Patients being treated for asthma may present with clinical features of eosinophilic pneumonia or eosinophilic granulomatosis with polyangiitis (EGPA). These events may be associated with the reduction of oral corticosteroid therapy. Healthcare providers should be alert to vasculitic rash, worsening pulmonary symptoms, cardiac complications, kidney injury, and/or neuropathy presenting in their patients with eosinophilia. Cases of eosinophilic pneumonia were reported in adults who participated in the asthma development program and cases of EGPA have been reported with DUPIXENT in adults who participated in the asthma development program as well as in adults with co-morbid asthma in the CRSwNP development program. Advise patients to report signs of eosinophilic pneumonia and EGPA. Consider withholding DUPIXENT if eosinophilic pneumonia or EGPA are suspected.
Acute Symptoms of Asthma or Chronic Obstructive Pulmonary Disease or Acute Deteriorating Disease: Do not use DUPIXENT to treat acute symptoms or acute exacerbations of asthma or COPD, acute bronchospasm, or status asthmaticus. Patients should seek medical advice if their asthma or COPD remains uncontrolled or worsens after initiation of DUPIXENT.
Risk Associated with Abrupt Reduction of Corticosteroid Dosage: Do not discontinue systemic, topical, or inhaled corticosteroids abruptly upon initiation of DUPIXENT. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a healthcare provider. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Patients with Co-morbid Asthma: Advise patients with co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians.
Psoriasis: Cases of new-onset psoriasis have been reported with the use of DUPIXENT for the treatment of atopic dermatitis and asthma, including in patients without a family history of psoriasis. In postmarketing reports, these cases resulted in partial or complete resolution of psoriasis with discontinuation of dupilumab, with or without use of supplemental treatment for psoriasis (topical or systemic). Those who continued dupilumab received supplemental treatment for psoriasis to improve associated symptoms. Advise patients to report new-onset psoriasis symptoms. If symptoms persist or worsen, consider dermatologic evaluation and/or discontinuation of DUPIXENT.
Arthralgia and Psoriatic Arthritis: Arthralgia has been reported with the use of DUPIXENT with some patients reporting gait disturbances or decreased mobility associated with joint symptoms; some cases resulted in hospitalization. Cases of new-onset psoriatic arthritis requiring systemic treatment have been reported with the use of DUPIXENT. Advise patients to report new-onset or worsening joint symptoms. If symptoms persist or worsen, consider rheumatological evaluation and/or discontinuation of DUPIXENT.
Parasitic (Helminth) Infections: It is unknown if DUPIXENT will influence the immune response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, discontinue treatment with DUPIXENT until the infection resolves. Helminth infections (5 cases of enterobiasis and 1 case of ascariasis) were reported in pediatric patients 6 to 11 years old in the pediatric asthma development program.
Vaccinations: Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating DUPIXENT. Avoid use of live vaccines during treatment with DUPIXENT.
ADVERSE REACTIONS:Most common adverse reactions are:
Please see accompanying full Prescribing Information
Atopic Dermatitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 6 months and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids.
Asthma: DUPIXENT is indicated as an add-on maintenance treatment of adult and pediatric patients aged 6 years and older with moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid dependent asthma. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm or status asthmaticus.
Chronic Rhinosinusitis with Nasal Polyps: DUPIXENT is indicated as an add-on maintenance treatment in adult and pediatric patients aged 12 years and older with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).
Eosinophilic Esophagitis: Dupixent is indicated for the treatment of adult and pediatric patients aged 1 year and older, weighing at least 15 kg, with eosinophilic esophagitis (EoE).
Prurigo Nodularis: DUPIXENT is indicated for the treatment of adult patients with prurigo nodularis (PN).
Chronic Obstructive Pulmonary Disease: DUPIXENT is indicated as an add-on maintenance treatment of adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm.
Chronic Spontaneous Urticaria: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 12 years and older with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. Limitations of Use: DUPIXENT is not indicated for treatment of other forms of urticaria.
Bullous Pemphigoid: DUPIXENT is indicated for the treatment of adult patients with bullous pemphigoid (BP).
CONTRAINDICATION: DUPIXENT is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients.
WARNINGS AND PRECAUTIONSHypersensitivity: Hypersensitivity reactions, including anaphylaxis, acute generalized exanthematous pustulosis (AGEP), serum sickness or serum sickness-like reactions, angioedema, generalized urticaria, rash, erythema nodosum, and erythema multiforme have been reported. A case of AGEP was reported in an adult subject who participated in the bullous pemphigoid development program. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue DUPIXENT.
Conjunctivitis and Keratitis: Conjunctivitis and keratitis occurred more frequently in AD, COPD, and BP subjects who received DUPIXENT versus placebo, with conjunctivitis being the most frequently reported eye disorder in AD. Conjunctivitis also occurred more frequently in adult CRSwNP and PN subjects who received DUPIXENT compared to those who received placebo. Conjunctivitis and keratitis have been reported with DUPIXENT in postmarketing settings, predominantly in AD patients. Some patients reported visual disturbances (e.g., blurred vision) associated with conjunctivitis or keratitis. Advise patients or their caregivers to report new-onset or worsening eye symptoms. Consider ophthalmological examination for patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis, as appropriate.
Eosinophilic Conditions: Patients being treated for asthma may present with clinical features of eosinophilic pneumonia or eosinophilic granulomatosis with polyangiitis (EGPA). These events may be associated with the reduction of oral corticosteroid therapy. Healthcare providers should be alert to vasculitic rash, worsening pulmonary symptoms, cardiac complications, kidney injury, and/or neuropathy presenting in their patients with eosinophilia. Cases of eosinophilic pneumonia were reported in adults who participated in the asthma development program and cases of EGPA have been reported with DUPIXENT in adults who participated in the asthma development program as well as in adults with co-morbid asthma in the CRSwNP development program. Advise patients to report signs of eosinophilic pneumonia and EGPA. Consider withholding DUPIXENT if eosinophilic pneumonia or EGPA are suspected.
Acute Symptoms of Asthma or Chronic Obstructive Pulmonary Disease or Acute Deteriorating Disease: Do not use DUPIXENT to treat acute symptoms or acute exacerbations of asthma or COPD, acute bronchospasm, or status asthmaticus. Patients should seek medical advice if their asthma or COPD remains uncontrolled or worsens after initiation of DUPIXENT.
Risk Associated with Abrupt Reduction of Corticosteroid Dosage: Do not discontinue systemic, topical, or inhaled corticosteroids abruptly upon initiation of DUPIXENT. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a healthcare provider. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Patients with Co-morbid Asthma: Advise patients with co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians.
Psoriasis: Cases of new-onset psoriasis have been reported with the use of DUPIXENT for the treatment of atopic dermatitis and asthma, including in patients without a family history of psoriasis. In postmarketing reports, these cases resulted in partial or complete resolution of psoriasis with discontinuation of dupilumab, with or without use of supplemental treatment for psoriasis (topical or systemic). Those who continued dupilumab received supplemental treatment for psoriasis to improve associated symptoms. Advise patients to report new-onset psoriasis symptoms. If symptoms persist or worsen, consider dermatologic evaluation and/or discontinuation of DUPIXENT.
Arthralgia and Psoriatic Arthritis: Arthralgia has been reported with the use of DUPIXENT with some patients reporting gait disturbances or decreased mobility associated with joint symptoms; some cases resulted in hospitalization. Cases of new-onset psoriatic arthritis requiring systemic treatment have been reported with the use of DUPIXENT. Advise patients to report new-onset or worsening joint symptoms. If symptoms persist or worsen, consider rheumatological evaluation and/or discontinuation of DUPIXENT.
Parasitic (Helminth) Infections: It is unknown if DUPIXENT will influence the immune response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, discontinue treatment with DUPIXENT until the infection resolves. Helminth infections (5 cases of enterobiasis and 1 case of ascariasis) were reported in pediatric patients 6 to 11 years old in the pediatric asthma development program.
Vaccinations: Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating DUPIXENT. Avoid use of live vaccines during treatment with DUPIXENT.
ADVERSE REACTIONS:Most common adverse reactions are:
Please see accompanying full Prescribing Information
References: